Conjugated Polymers
ORGANIC MATERIALS FOR PHOTOVOLATICS AND THERMOELECTRICS
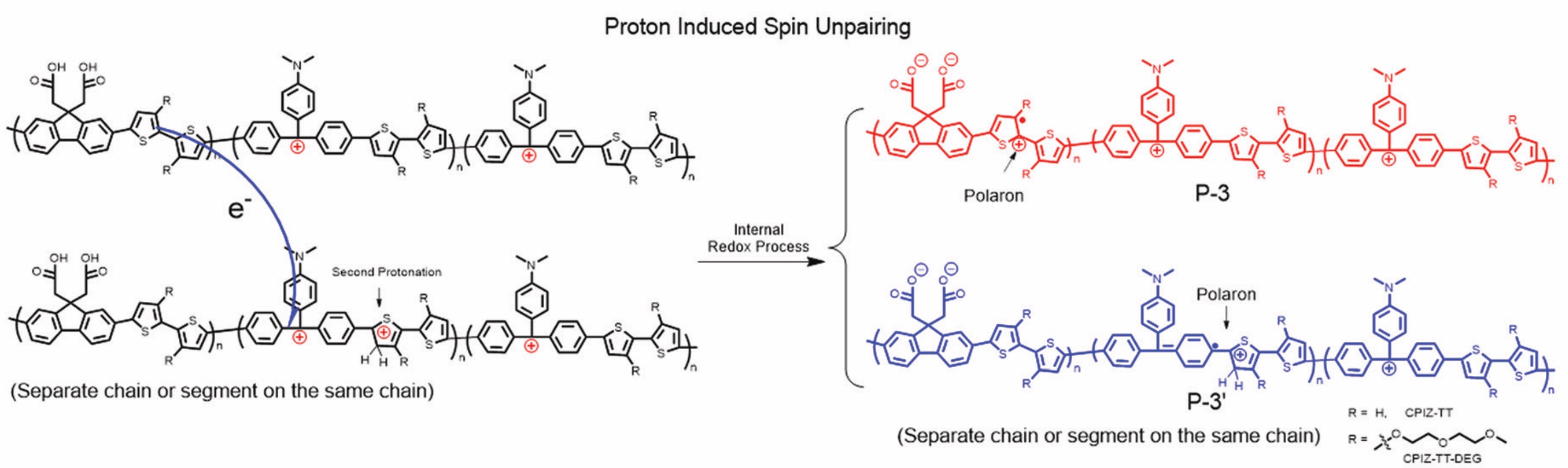
We are investigating new ideas for organic materials for the next generation of organic thermoelectric generators and excitonic solar cells.
Certum Est, Quia Impossibile

ORGANIC MATERIALS FOR PHOTOVOLATICS AND THERMOELECTRICS
We are investigating new ideas for organic materials for the next generation of organic thermoelectric generators and excitonic solar cells.

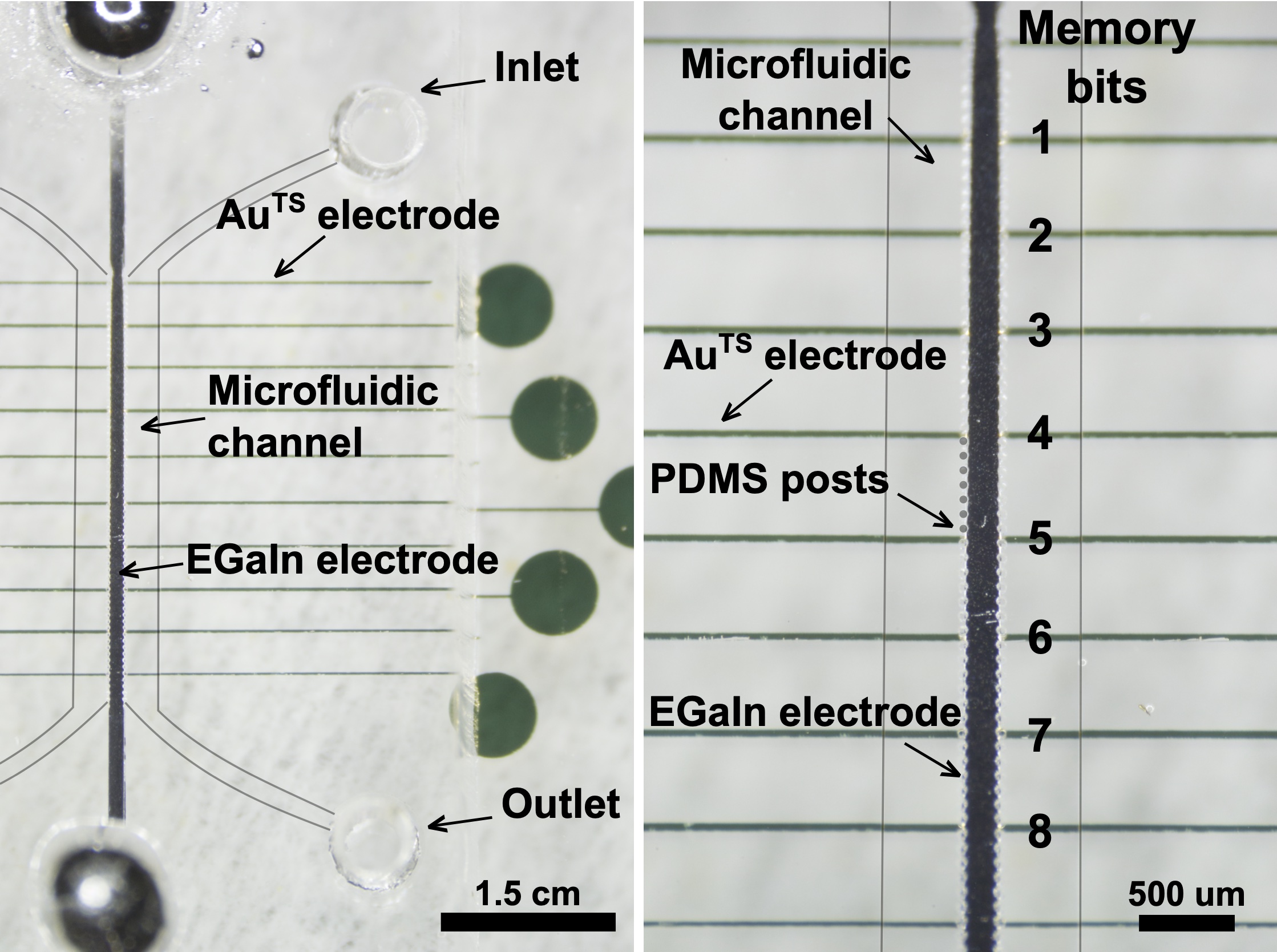
SELF-ASSEMBLED MOLECULAR JUNCTIONS
We are developing new ways to contact ensembles of molecules on the micro scale, and collections of single molecules on the nanometer scale using EGaIn, Nanoskiving and soft lithography.
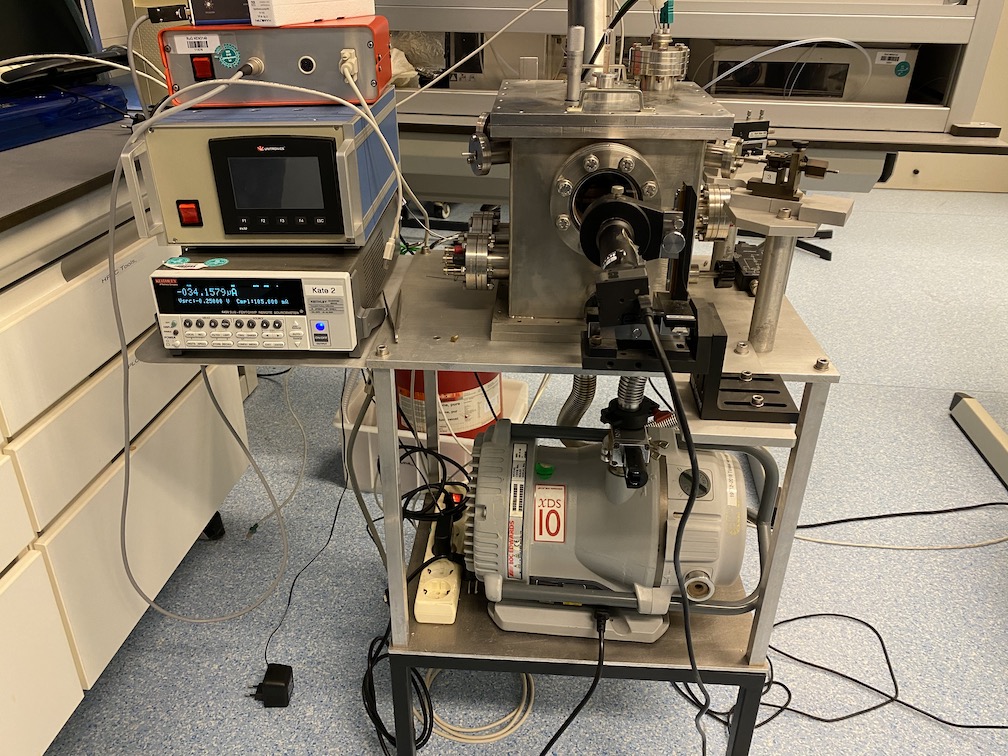
MICRO/NANOFABRICATION FOR UNCONVENTIONAL ELECTRONICS
We are developing tools and techniques for leveraging self-assembly, organic synthesis and rapid prototyping to fabricate electronic devices with unique functionality.